The Potential of Psychedelics
#96: On the evolving landscape of psychedelic drugs in medical applications.
One of the big new-era ideas that has gained popularity in science, popular culture, and the stock market in recent years, is the medical potential of psychedelics — using drugs like LSD, MDMA, or psilocybin in the treatment of a variety of medical conditions.
As the story goes, many of these compounds were originally synthesized for medical purposes, and naturally occurring psychedelics have been used in alternative medicine for centuries. Indeed there was fertile research into these drugs’ potential throughout the mid-20th century. But concerns of abuse, side effects, and social backlash ultimately resulted in the banning of these substances, halting medical research in the process.
But as attitudes towards these drugs have evolved, this dormant field of exploration has seen a modern revival with seemingly promising results. These ideas have been further popularized throughout the media — in headlines and on Netflix.
Enthusiasm for a new category of therapeutics has spawned a litter of biotechnology companies seeking to bring such drugs through clinical trials and ultimately to market. At peak enthusiasm, there were even two separate ETFs launched to track baskets of such companies — the Horizons Psychedelic Stock Index (PSYK) and the AdvisorShares Psychedelics ETF (PSIL).
But at least in the market’s eye, psychedelics’ spring revival peaked along with the meme stocks in early-2021 and has since given way to a winter of discontent, as lofty potential has succumbed to the here-and-now. Currently, no traditional psychedelic1 has been approved by the FDA for medical use in the United States. Investment performance in clinical stage psychedelic research has been… lackluster.
But even as stocks fall, the research continues.
In 2023, the FDA provided new guidelines for psychedelic drug trials, showing an increasingly supportive regulatory regime. This year, we may see the first FDA approval of a psychedelic — using MDMA with psychotherapy in the treatment of post-traumatic stress disorder (PTSD). And just in the past two weeks, two separate drugs were granted “breakthrough therapy designation” by the FDA following positive Phase II results, which would help to expedite the drugs approval process to the extent they continue to show safety and efficacy in Phase III trials.
In other words, we are closer today to seeing the fruit of this research, and yet most companies in the space are still discounted as the dregs of a bubble-past.
Important caveats. I’m not a doctor, nor do I have experience evaluating clinical drug development. This article doesn’t attempt to comprehensively address concerns of side effects or unravel the thorny history of prescribing drugs with potential for abuse. Nor is this article intended to be a stock pitch, even if some of the information may be relevant to investors.
But, as a general matter I do find this particular intersection of finance, health, and science quite fascinating, and I think you may as well. Let’s dig in.
The Landscape
Presently, there are numerous clinical trials underway, sponsored by an array of biotechnology companies, testing the safety and efficacy of psychedelic drugs.
These trials typically involve pairing a specific drug candidate (formulation, dose, delivery method) against a specific diagnosed condition. The primary area of focus has been on treating mental health disorders such as post-traumatic stress disorder (PSTD), major depressive disorder (MDD), treatment resistant depression (TRD), or general anxiety disorder (GAD), but earlier stage research expands the window of exploration to include substance abuse, obsessive compulsive disorder (OCD), anorexia, and more.
Below is a select list of psychedelic drugs in moderately advanced clinical trials.
Generally, treatment involves administering the drugs to patients in a controlled and accommodative environment under the supervision of a psychiatrist. There is also a range of other important variables. In some trials, the treatment sessions are accompanied by talk-therapy, while others are not. Some are being tested in combination with existing psychiatric medication, while others are tested in isolation. Dosages and repetition vary case by case.
As with all FDA approvals, these drug trials involve three phases. First, testing the safety and tolerability of the compound, second, measuring its effectiveness in treating a diagnosis, and third, replicating the results in larger studies while also evaluating negative outcomes.
The efficacy of the treatment is determined by changes in symptoms in follow-up visits, as measured by standardized rubrics such as the Montgomery–Åsberg Depression Rating Scale (MADRS), generally several weeks after treatment. Longer-term check-ins also test the durability of effect.
While researchers have a narrow understanding of the neural pathways in play — they understand the specific receptors the compounds interact with — it is less clear exactly how these drugs produce results. But the broad understanding is that these drugs can stimulate new ideas, widen perspectives, and change patterns of thinking. And while the acute effects of the drugs are limited to several hours, the impact to a patient’s thought process can be long lasting. In this sense, treatment can penetrate the rumination and rut of inescapable negative thoughts that often typify forms of depression, or other anti-social behavior.
Here is the truly interesting hope — that by allowing people to think in new ways, these drugs may actually get closer to addressing the cause of these mental diseases, rather than merely addressing symptoms. Further, there is the hope that even single treatment sessions can potentially provide long-lasting benefits. A growing body of clinical data supports these ideas. Here are some worth noting…
Therapies
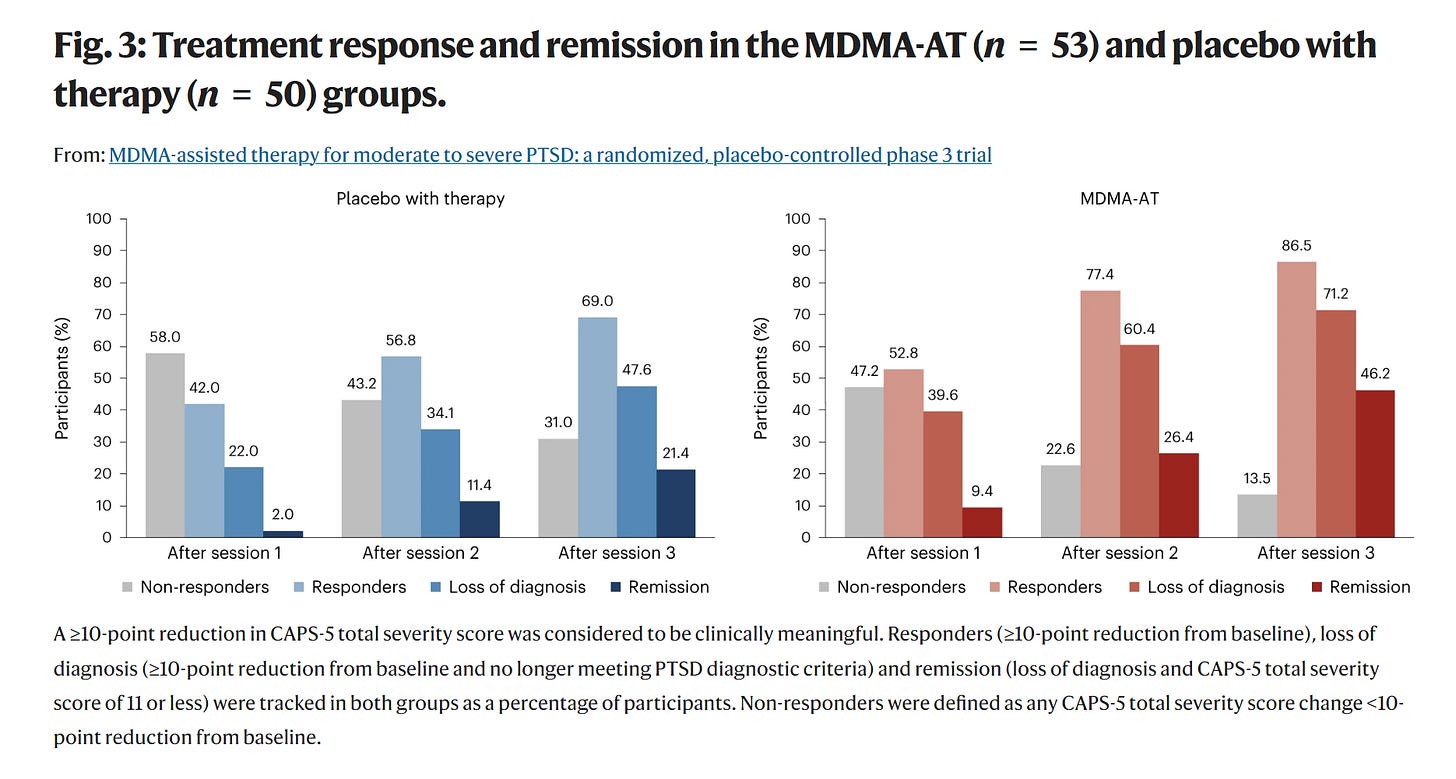
MDMA-AT for Post-Traumatic Stress Disorder: The most advanced drug in development is MDMA for the treatment of PTSD, sponsored by Lykos Therapeutics (private), a newly formed entity controlled by the Multidisciplinary Association for Psychedelic Studies (MAPS). In its Phase III study published in Nature, patients who received MDMA during three separate eight-hour therapy sessions showed a significant improvement in symptoms vs. placebo.
The study showed 86.5% of patients demonstrated a positive response to treatment, vs. 69% in placebo. More impressive, 46.2% of patients were deemed to be in remission after the third session, compared to 21.4% in the placebo group.
Side effects were characterized as mild to moderate, and basically describe the physiological effects of MDMA - racing heart, nausea, dizziness etc. But encouragingly, the dropout rate of the MDMA group in study was incredibly low at just 1.9%, compared to 15.7% in placebo, and >50% in unrelated PTSD studies testing prolonged exposure and cognitive processing therapy. This suggests a high degree of practical tolerability despite the noted side effects.
Lykos/MAPS submitted its New Drug Application (NDA) to the FDA in December 2023 and was accepted for priority review on February 9, 2024. A ruling on the drug is targeted for August 2024. If accepted, it would mark the first traditional psychedelic treatment approved in the United States.
Psilocybin for Treatment Resistant Depression (TRD)
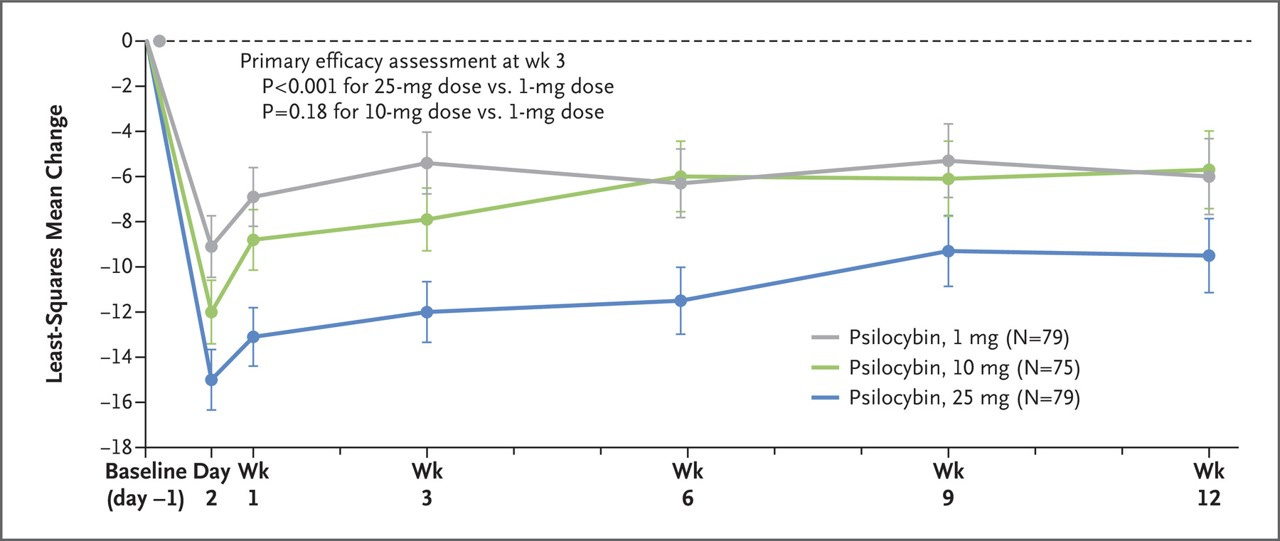
Compass Pathways Inc. (NASDAQGS: CMPS) is one of several companies advancing research of psilocybin for various forms of depression. Currently, the company is conducting Phase III trails on its COMP360 compound based on positive results from its Phase II trials which were published in the New England Journal of Medicine in 2022.
The previous trails involved a single dose of either 1mg, 10mg, or 25mg psilocybin in a therapy guided session. Individuals were weaned off of other medication prior to the trial. The results showed a material and durable improvement in symptoms in the 25mg cohort, but not the 1mg (effectively placebo) or 10mg cohorts.
While promising results on average, the study also had noted drawbacks. Beyond the expected side-effects on the day of treatment, there were also serious effects in some participants that evolved in the days and weeks after, including suicidal ideation. These side effects were more prevalent in the higher dosage cohorts, suggesting a link to the drug itself.
The company is currently in the process of conducting Phase III study, with topline results expected in late 2024, and full data in 2025.
LSD for General Anxiety Disorder (GAD):
Just last week, Mind Medicine Inc. (NASDAQ: MNMD), released the full results of its Phase IIb study of its formulation of LSD, Lysergide D-Tartrate or MM120 for treatment of GAD. Participants were given either 25μg, 50μg, 100μg, 200μg of MM120 or a placebo. The study was differentiated in that it did not include psychotherapy beyond simple supervision, seeking to emphasize the role of the compound itself. Participants were also weaned off other medications prior to the trial.
The trial produced positive results in the primary endpoint (HAM-A score) as well as secondary endpoints (MADRS score). All dosages showed efficacy vs. placebo, with 100μg dosage appearing to be optimal. In that dose, 65% of participants showed response (vs. 31% in placebo) and 48% showed remission (vs. 21% in placebo) after 12 weeks.
For a frame of reference, the company reports that these results are over twice as effective as existing medications including benzodiazepines and SSRIs.
Based on these results, last week, the FDA granted the drug “breakthrough therapy designation”, which helps expedite ongoing trails and approvals. Phase III trials are expected to begin in the second half of 2024. On the back of these positive developments, the company was able to raise $175 million in new equity capital, de-risking the funding needs for the immediate future.
Psilocybin for Major Depressive Disorder (MDD)
Finally, another candidate worth watching comes from Cybin, Inc. (ARCA: CYBN). Its drug candidate CYB003 is a deuterated psilocybin analog, which this week also gained “breakthrough therapy designation” from the FDA.
Its Phase II study released this week, tested two doses of psilocybin at 12mg or 16mg, four weeks apart. A placebo group from the first session received an active dose in the second session. Importantly, the trial was adjunctive — meaning that patients did not stop their existing medications — and sets the course for this drug to be used in conjunction with other medications if ultimately approved, a significant differentiation.
The trial showed significant and durable effects measured by a reduction in MADRS scores through four months, albeit with a smaller sample size than the other studies noted above.
CYB003 also produced a significantly stronger response than existing medications, and an even greater result when compared to existing adjunctive medications.
These results allowed Cybin to raise an additional $150 million of equity in a private placement this week, which will again help support the company’s funding needs through Phase III trials which are expected to begin in 2H 2024.
Commercialization
FDA approval is of course a major threshold, but what happens after? While there is no precise template for this new class of drug, the best analog for a commercial roll-out is likely Johnson and Johnson’s (JNJ) Spravato.
Spravato, the trade name of esketamine, is a ketamine-based nasal spray first approved by the FDA in 2019 for Treatment Resistant Depression. While ketamine is a dissociative hallucinogen and sometimes considered a psychedelic, it is more commonly understood as an sedative and anesthetic. Spravato is administered to patients during supervised sessions at specialized clinics, and given the similarities between treatment method and targeted condition, it seems to provide a roadmap for how psychedelics may eventually take commercial form.
Spravato has been a success. It currently is the fastest growing drug in JNJ’s portfolio2, with annualized sales figures reaching over $820 million as of 4Q 2023, up 74% year over year. At its current growth rate, Spravato may cross $1 billion in sales in 2024. In its investor materials, JNJ considers Spravato to be a $1bn - $5bn opportunity, in terms of peak annual sales.
While many unknowns exist — approvals, competition, pricing, etc. — it does seem like there is a significant opportunity ahead for the first-movers that can successfully bring drugs to market. And while at their peak, many of these biotechs garnered multi-billion valuations, today most trade well below $500 million in market cap, suggesting there could be upside for winners.
Conclusions
Pulling back the curtain just a bit, I’m very aware of the debilitating effects of mental disorders and depression for affected individuals and their families. Current treatment options often seem ineffective, frustrating, and many times come with their own negative side effects.
I’m also aware that, for better or worse, psychedelics can create powerful and lasting impressions in the mind. At least in concept, there does seem to be a real opportunity for genuinely transformative experiences for folks suffering from these conditions. Even if the research ultimately doesn’t pan out, the initial results surely justify ongoing exploration.
But power comes with responsibility. If psychedelics help “re-wire” people’s thoughts, they are not guaranteed to do so positively. In recreational settings, it is not uncommon for usage to prompt depression or anxiety. In studies of recreational users, 24% reported negative psychological symptoms lasting one week or more, including fear, anxiety, depression and paranoia, with 10% reporting negative symptoms a year afterwards. Understanding the risk of these adverse outcomes must be part of the calculus.
And even as I mentioned Spravato as a business success, I am inherently wary of specialized clinics that dispense addictive sedatives. Fairly or not, to me, it echoes of the pain management clinics that were instrumental in promulgating the opiate crisis, which continues to ravage this country today. Evaluating the potential for dependence and abuse must be a major consideration.
Even in the most optimistic scenario, it will be years before most these treatments become widely available. And it remains to be seen which, if any, ultimately pass the muster of large scale replicability, safety and FDA approval.
But there is certainly potential.
Potential for genuine improvement in the lives of patients, potential for the success of pioneering companies, and potential for us collectively to better understand the magic of the mind.
Mindfully,
The Last Bear Standing
Excluding ketamine, which is sometimes considered a psychedelic, and is discussed in more detail below.
At least of drugs specifically itemized in its quarterly reporting framework.










Got a little bit dizzy reading this ;)
Loved it!
Thanks for this Mr. Bear!